Data Analytic Services
Statisticians and writers at B2S possess a wide range of experience at a variety of pharmaceutical companies. Collaborations between the statistics team, clinical scientists, data managers and medical or technical writers allow us to generate high-quality data and interpretation in a timely fashion. Some key capabilities include
Immunogenicity – Cut Point Determination, Consulting Support, Writing Support
Cut Point Statistics
-
- Develop study design and plate maps for running samples
- Create and review statistical and/or cut point sections of protocols, validation plans, sample analysis plans
- Create and review analysis plans for cut point determination
- Program datasets and output of cut points, sensitivity, projected LPC
- Write cut point reports, manuscripts, presentations
Consulting and Writing Support
-
- Prepare Immunogenicity Risk Assessment documents
- Prepare clinical immunogenicity report for inclusion in clinical study report
- Integrated Summary of Immunogenicity: prepare analysis plan, program outputs, write report
- Assist in answering questions from global regulatory authorities
- Critical reagent consultation
Immunogenicity Tools
B2S has developed an on-line tool to calculate screening, confirmatory, and titer cut points.
General Statistical Support
- Develop protocols and study designs; sample size and power calculations
- Create and review statistical analysis plans
- Interim analysis and assessment committees: create materials, interpret results in consultation with medical team members
- Prepare analyses for study reports, manuscripts or presentations, periodic safety reports
Pharmacokinetics
PK/TK Data Analysis and Consulting Support
The applied science fields of pharmacokinetics, toxicokinetics, pharmacodynamics, clinical pharmacology, and bioanalytical sciences are inextricably connected and are core areas of expertise for our consultants. Successful development and commercialization of today’s biotechnology and conventional drugs requires the careful planning, execution and analysis of a series of toxicology studies during preclinical evaluation and clinical pharmacology studies conducted in phase I to phase IV. Data from such investigations take a prominent place in product labeling, provide an underpinning for the mechanism of action, establish vital exposure-response relationships, and define dosing requirements in different populations. At the heart of all these studies are robust validated bioanalytical measurements of the drug substance in animal or human tissues and the application of state-of-the-art pharmacokinetic or pharmacodynamic analyses using specific models to assess and summarize the important findings. The efficiency of drug development can be increased by leveraging PK/PD analyses to understand complex dose-exposure-response time relationships.
Our consultants can help develop innovative strategies for implementing decisive studies that enhance the toxicological and clinical pharmacological evaluation of drugs leading to commercial success.
These services include:
- Non-GLP or GLP toxicokinetic evaluation for toxicology or ADME studies
Nonclinical TK and Clinical data analysis supported
- Exposure-dose-response relationships
- Accumulation and linearity assessment
- Individual animal or pooled data analyses – protocol specified analyses
- PK evaluation of phase I-IV clinical pharmacology and efficacy/safety studies
- Bioavailability, bioequivalence, drug interaction, and special population studies
Drug Development Support and Consulting
Drug Program Management – In House Drug development experts that lead programs from discovery to lead optimization and through clinical development
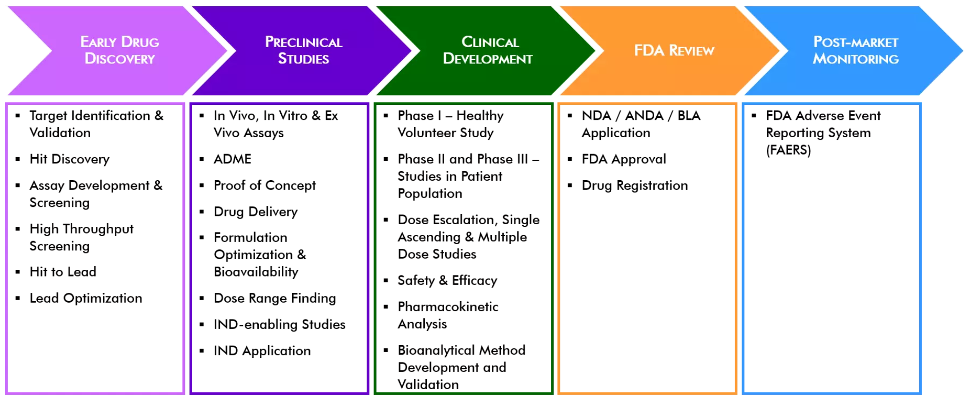
B2S Services Include:
- Developing non-clinical and clinical bioanalytical strategies supporting PK/TK and immunogenicity
- Drafting or reviewing bioanalytical methods for GLP and nonGLP studies
- Create assay and reagent Life Cycle management strategies to be consistent across programs
- Manage the development and implementation of a consistent and aligned assay strategy across programs
- Write and update immunogenicity risk assessments throughout drug development lifecycle
- Develop clinical phase appropriate and risk based statistical analysis plan for immunogenicity assessments
- Review PK and immunogenicity sections of Clinical Study Reports (CSR)
- General medical writing
- IND/BLA Plan and Submissions
- CRO Oversight
- Medical Writing