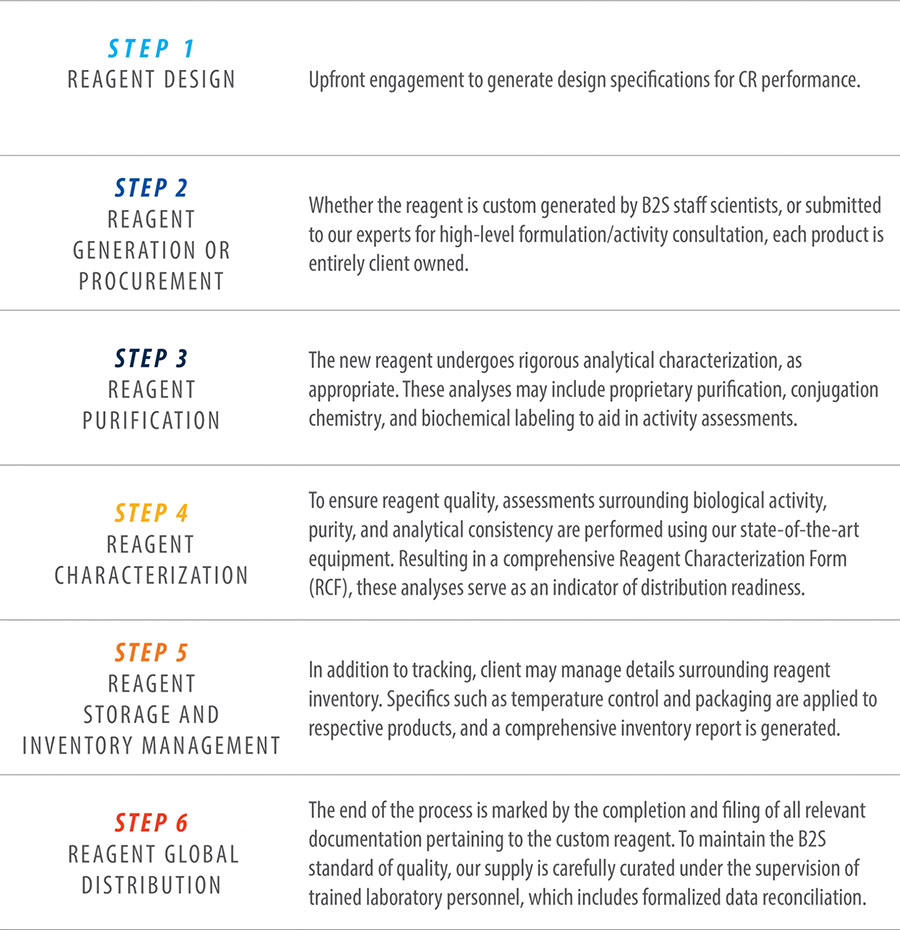
Lab Services
Custom Reagents
B2S Life Sciences has extensive expertise in the design, generation, characterization and life cycle management of custom, biological reagents. These custom reagents can be utilized to create robust assays for regulated bioanalysis to support evaluations of pharmacokinetics (PKs), toxicokinetic (TKs), immunogenicity of biotherapeutics and biomarkers.
Some of our unique critical reagent competencies include:
- Decades of experience in protein purification (antibodies, enzymes, and other structural proteins) using various chromatographic techniques.
- Access to several powerful biochemical and biophysical tools for detailed characterization of purified proteins and conjugates. Analysis of charge variants (cIEF, CZE, IEX), thermal stability (Tm, Tagg) using Unchained Labs Uncle platform, particle size distribution (DLS), binding kinetics by biolayer interferometry (Sartorius Octet/HTX), high resolution purity assessments (CE SDS-PAGE, HPLC, UHPLC) are all available to characterize your biologics.
- Capability to generate protein or peptide critical reagents conjugated to chemical tags (Biotin/Sulfo-TagTM/Florescence-tag). Antibody-drug conjugates (ADC), including labeled derivatives may also be prepared. Reagents may be prepared at small scale (≤1 mg) to scale-up production (≥ 100 mg) in sufficient quantity to avoid the need for producing new lots during in-study analysis of test samples and eliminate the risk of lot-to-lot variability.
- Generation and characterization of anti-drug antibodies (ADA) positive controls in Rabbits/Chickens/Guinea Pigs etc.
- Capability to screen and purify preexisting human anti-drug antibody (ADA) positive controls from the serum of individuals who are seropositive (e.g. Purification of Gene Therapy vector, Adeno-Associated Virus (AAV) specific antibodies from seropositive human serum).
- Purification and characterization of Biomarker reference standard to support biomarker and CLIA assessments. Capability to scale critical reagent production in sufficient quantity to avoid the need for producing new lots during In-study analysis of test samples.
- Design, production, and characterization of matched antibody pairs for optimization of PK/TK and quantitative biomarker assays.
- Cell Culture Supporting Reagent Generation and Neutralizing Ab Assays
- In-house generated custom Critical Reagents will be extensively biophysically and/or biochemically characterized for their intended use and a Reagent Characterization Form (RCF) will be available to the clients. The RCF includes but are not limited to the following: purity, concentration, label incorporation ratio, activity, titer, binding affinity, specificity, isotype (monoclonal/polyclonal antibody), and aggregation propensity.
- Long-term storage of reagents in temperature-controlled environment (ambient, 4, -20, -80°C)
- ‘First-of-a-kind’ inventory management tool (LCM+) enables clients with on-line secure access to their reagent critical information.
- Periodic retest dates to ensure quality and updating of RCF.
- Support for reagent management and global distribution.
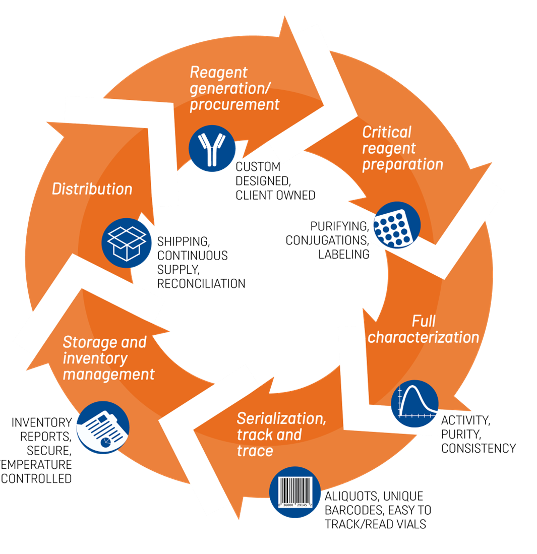
Our critical reagent design and development is a comprehensive technical collaboration between our experienced staff scientists and our external partners. Our focus is always on mutual success, with a reagent strategy meticulously tailored to specific client needs. Under the supervision of a technical expert, clients can expect to be guided in the following sequence:
Life Cycle Management
B2S Life Sciences is the only CRO that offers clients a wholly integrated solution for Life Cycle Management Program (LCM+) of custom critical reagents.
Assay Development and Qualification
Our Assay Development team designs and executes assays that complement our capabilities in custom critical reagents to enable our clients to efficiently make data-driven decisions for optimal analytical outcomes to support biotherapeutic drug development.
To realize this goal, B2S offers a range of services from drug discovery through nonclinical/clinical development. This includes early phase ADME investigations, reagent optimization, generation of optimal matched pair of antibodies, method qualification, and non-GLP sample analysis.
The range of bioanalytical methods includes assays to support PK/TK, detection and characterization of anti-drug antibody immunogenicity assays, assays to determination of novel biomarkers for assessments of pharmacodynamics.
These services include, but are not limited to:
- Reagent screening, binding kinetics, clone selection, Ab pair-selection, and epitope mapping/binning
- Quantitative method development
- PK/TK
- Biomarker
- Immunogenicity method development
- ADA
- Competitive LBA Nab
- Cell-based Nab
- Flow-based assays
- Coated assay plate generation/development (i.e. ELISA, ECLIA, etc.)
- Critical Reagent Pack (Pharma-Grade Kit) development
- Chemistry, Manufacturing, and Controls (CMC) supporting assay development (e.g. Stability-indicating Drug Product Release Assay)
- Oversight of method transfer to a GLP contract research organization for sample analysis
- Exploratory and/or Non-GLP sample analysis
- Negative matrix pool screening
- Platform screening/conversion
- Drug Stability Comparisons (e.g. Buffer/Matrix half-life assessments of Drug Candidate(s)/Drug Products versus native endogenous molecule, precursors, and/or biosimilars
- Discovery & Lead Optimization Assay Support
- ADA and NAB Assays (hyperlink back to immunogenicity)
GLP Sample Analysis Lab Capabilities
The production lab at B2S Life Sciences has established itself as a GxP compliant lab and further enhances the support we can provide our clients. This lab is fully equipped to receive method transfers both internally and externally, validate methods, and support non-clinical and clinical programs in support of regulatory filings.
The production lab handles quantitative PK/TK as well as biomarker methods that support regulatory filing. Additionally, the lab performs full and partial validations of ADA methods that include screening, confirmatory, titration, domain specificity, and neutralizing antibody assays.
B2S Life Sciences can validate method using a variety of platforms including Electrochemiluminescence, Colorimetric ELISA, Luminometric ELISA, and Luminex bead-based methods
Cell Culture and Neutralizing Antibody Assays
At B2S Life Sciences we utilize cell culture in three major applications.
One application is the production of recombinant proteins in mammalian cells. In short, the cells are employed to produce critical reagents to meet the needs of our clients. Cell lines can be transiently or stably transfected to produce critical reagents, in order to ensurea continuous supply. This allows us to provide consistent, high-quality products for our clients.
Initial steps include creation and/or scale-up production of expression constructs with DNA encoding target recombinant proteins. We can also produce AAV (Adeno-associated virus) in large scale to support gene therapy.
The second major use for our cell culture facilities is cell-based assays. These assaysare performed in a separate facility that is dedicated for this purpose. Cell-based assays include but are not limited to assessments for neutralizing antibodies (including those that neutralize SARS-CoV-2), cell viability, apoptosis/necrosis, proliferation, and cytotoxicity, as well as immune cell isolation, separation, and characterization, etc. Multi-parameter flow cytometry analysis is available for assessments of immune cell phenotype, activation, differentiation, and other functional testing.
The final major application is reagent development via phage display technology (performed in conjunction with Tango Biosciences). Formats available for biopanning for binders of clients’ targets include both scFv and monobodies formed from a human fibronectin type III scaffold. Affinity maturation via directed molecular evolution is also an option.